High-entropy materials
Melting simulations of high-entropy carbonitrides by deep learning potentials (Sci. Rep., 14, 28678 (2024)) PDF
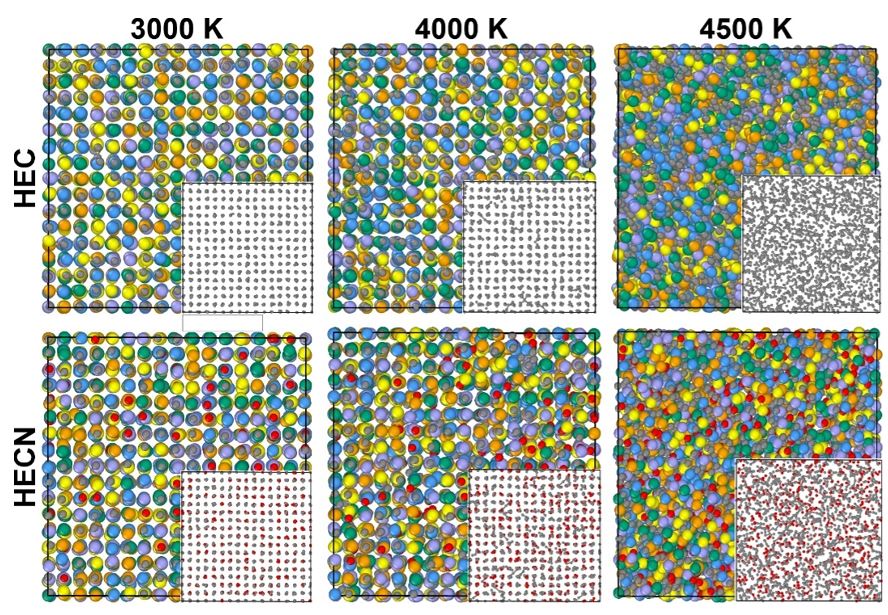
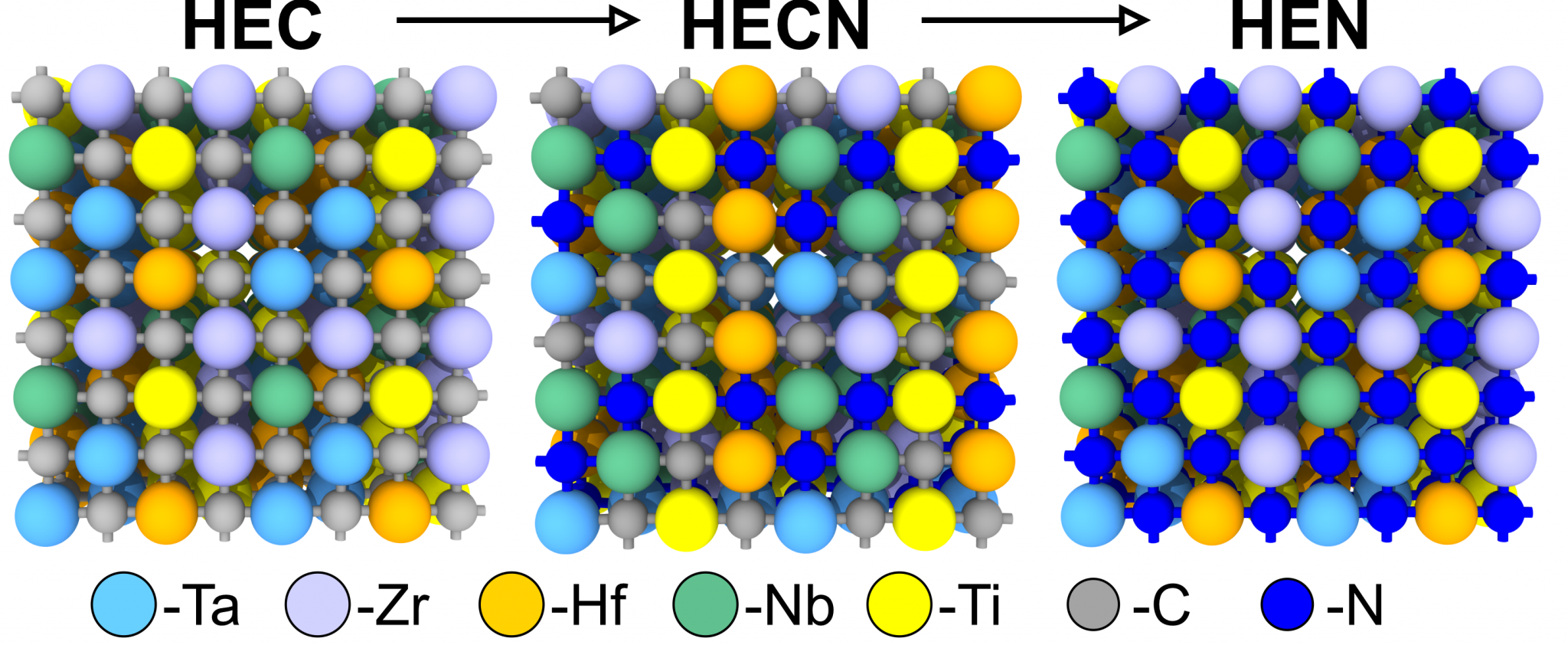
The melting temperature is a crucial property of materials that determines their potential applications in different industrial fields. In this study, we used a deep neural network potential to describe the structure of high-entropy (TiZrTaHfNb)CxN1−x carbonitrides (HECN) in both solid and liquid states. This approach allows us to predict heating and cooling temperatures depending on the nitrogen content to determine the melting temperature and analyze structure changes from atomistic point of view. A steady increase in nitrogen content leads to increasing melting temperature, with a maximum approaching for 25% of nitrogen in the HECN. A careful analysis of pair correlations, together with calculations of entropy in the considered liquid phases of HECNs allows us to explain the origin of the nonlinear enhancement of the melting temperature with increasing nitrogen content. The maximum melting temperature of 3580 ± 30 K belongs to (TiZrTaHfNb)C0.75N0.25 composition. The improved melting behavior of high-entropy compounds by the addition of nitrogen provides a promising way towards modification of thermal properties of functional and constructional materials.
Synthesis of high-entropy Ti-Zr-Nb-Hf-Ta carbides and carbonitrides in high-speed arc discharge plasma jet (J. All. Comp., 1010, 5, 177178 (2025)) PDF
The interest in high-entropy materials has increased rapidly in recent decades due to their applications in various fields such as environmental barrier coatings, superhard and wear resistant coatings, nuclear energy, batteries, catalysts, thermoelectrics, supercapacitors, biocompatible structures, and microelectronics. In the present work, comprehensive theoretical and experimental studies are carried out to discover a new way to prepare high-entropy ceramic nanopowders of carbides and carbonitrides of IV-V transition metals. The possibility of (TiZrNbHfTa)CxNy formation is investigated using both ab initio and machine learning approaches. The chosen single-stage plasma dynamic technique allowed us to synthesize high-entropy carbide TiZrNbHfTaC5 and the corresponding carbonitrides (N up to 8 wt%) in the form of single-crystalline nanoparticles. By varying the experimental system conditions, we demonstrate not only the production of pure powders, but also the ability to apply different precursors, including pure metals and their oxides. The presented technique provides a simple and universal way to produce high-entropy nanomaterials and opens the door to the synthesis of many functional ceramic powders composed of other carbonitrides with selective nitrogen content.
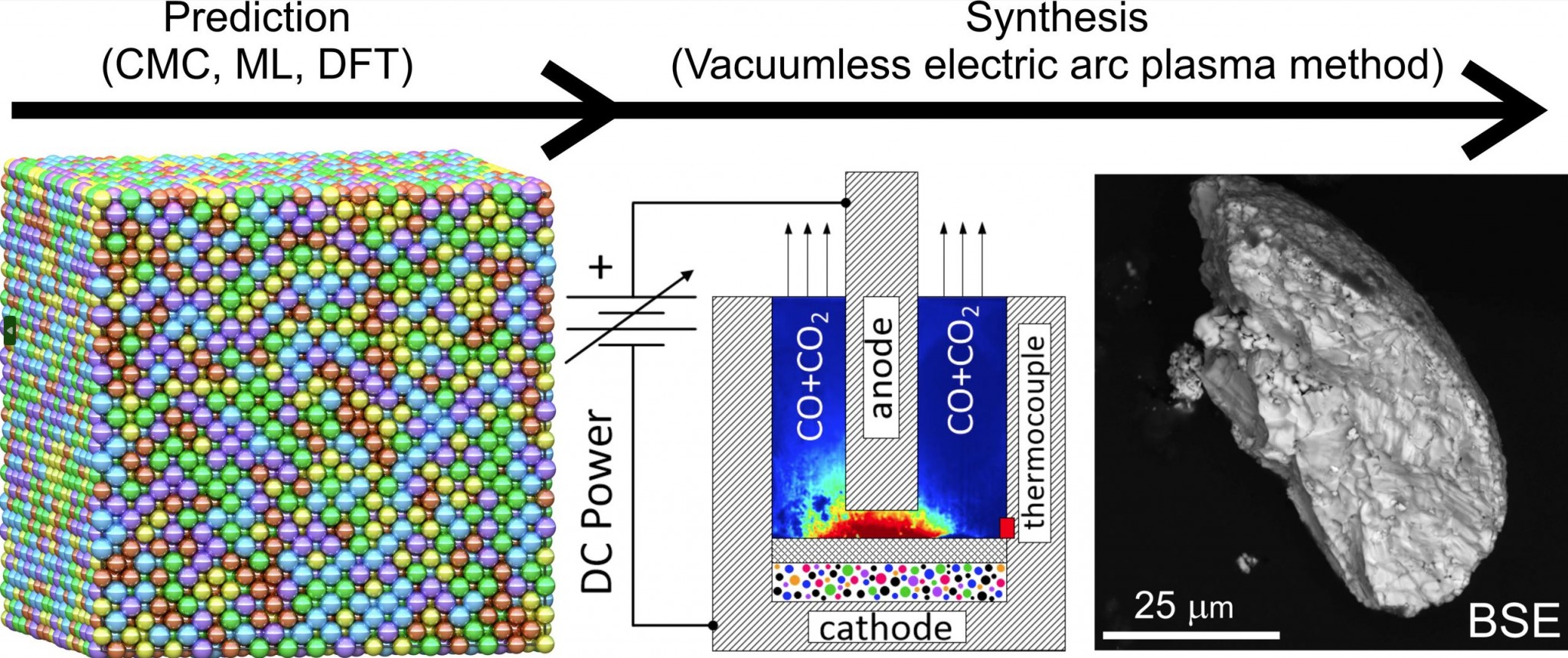
Machine learning-driven synthesis of TiZrNbHfTaC5 high-entropy carbide (npj Comp. Mat. 9, 7, (2023)) PDF
Synthesis of high-entropy carbides (HEC) requires high temperatures that can be provided by electric arc plasma method. However, the formation temperature of a single-phase sample remains unknown. Moreover, under some temperatures multi-phase structures can emerge. In this work, we developed an approach for a controllable synthesis of HEC TiZrNbHfTaC5 based on theoretical and experimental techniques. We used Canonical Monte Carlo (CMC) simulations with the machine learning interatomic potentials to determine the temperature conditions for the formation of single-phase and multi-phase samples. In full agreement with the theory, the single-phase sample, produced with electric arc discharge, was observed at 2000 K. Below 1200 K, the sample decomposed into (Ti-Nb-Ta)C, and a mixture of (Zr-Hf-Ta)C, (Zr-Nb-Hf)C, (Zr-Nb)C, and (Zr-Ta)C. Our results demonstrate the conditions for the formation of HEC and we anticipate that our approach can pave the way towards targeted synthesis of multicomponent materials.