New materials for catalysis
According to ESG initiative new materials for carbon capture and storage (CCS) are required. Multi-component, bimetallic, and high-entropy compounds (carbides, borides, nitrides etc.) are promising candidates for applications as catalysts and have not systematically studied yet.
Adsorption properties of crystalline and amorphous PdIr nanoparticles. A systematic first-principles study (J. Catalysis, 447, 116102 (2025))
The great interest in metal nanoparticles is due to the fact that the transition from micro to nano size leads to huge changes in the physical and chemical properties of the material. The local atomic structure and composition can significantly influence the properties of nanoparticles. First-principles calculations are used to study the influence of the structure and chemical ordering of IrPd nanoparticles on the electronic properties, charge distribution and adsorption energy of O, H, CO, NO, OH. Three different types of PdIr bimetallic nanoparticles with different chemical ordering are considered, namely Pd-core/Ir-shell (Pd@Ir), Ir-core/Pd-shell (Ir@Pd) and bimetallic alloy (Pd-Ir) particles consisting of 79 and 321 atoms with fcc and amorphous structures. The electronic and adsorption properties of the proposed nanoparticles are extensively studied in terms of their ability to adsorb O, H, CO, NO, OH, which opens up the possibility of fine-tuning their properties by modifying the atomic structure and composition. By adjusting the core–shell ratio, the adsorption energy on the nanoparticle surface can be fine-tuned, especially in fcc nanoparticles. This results in a narrower range of adsorption energies, which cannot be achieved with bimetallic alloys. In the case of amorphous nanoparticles, the adsorption energy is highly variable because there are many non-equivalent adsorption sites on the surface.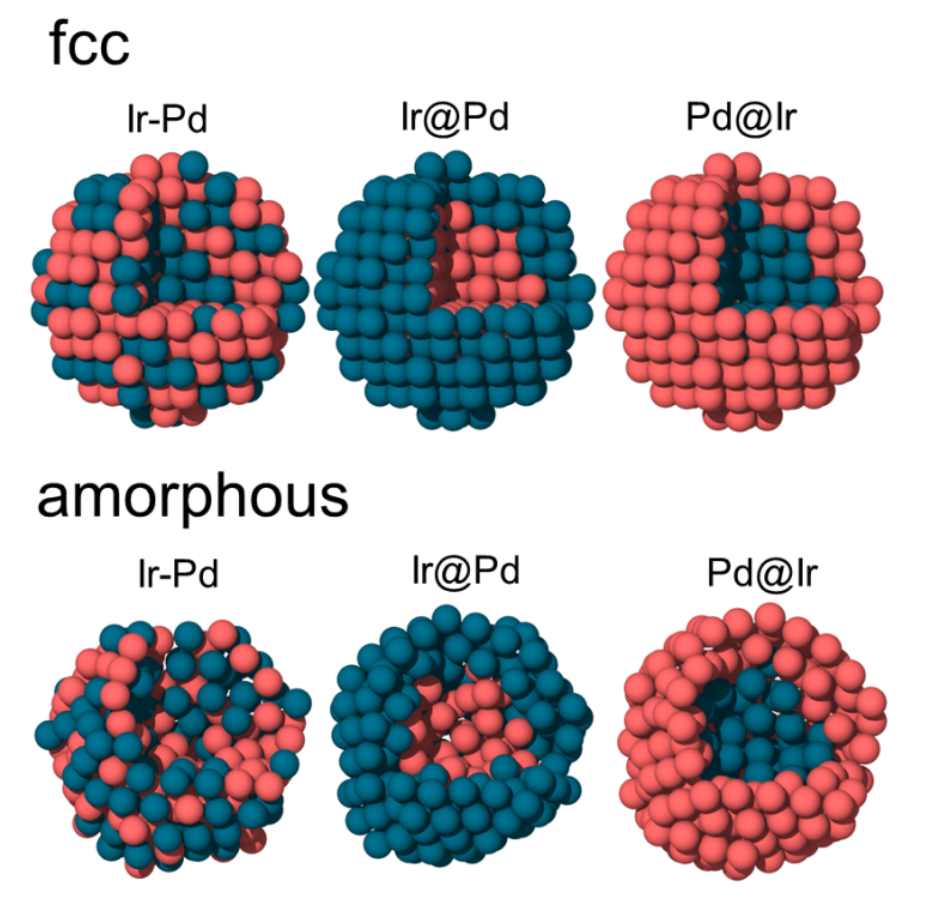
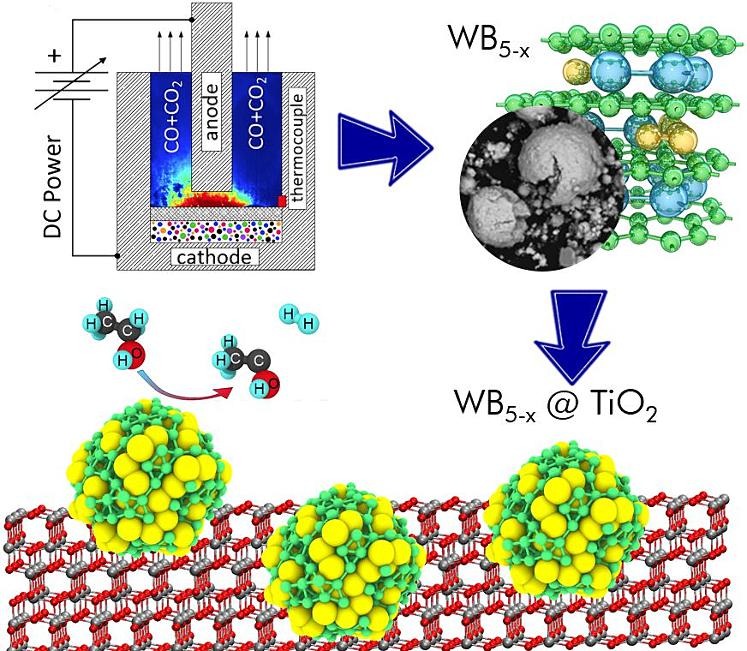
Application of tungsten pentaboride as cocatalyst for photocatalytic H2 evolution and CO2 reduction (Appl. Surf. Sci. 2024, 661, 160095) PDF
Catalytic reactions play an important role in modern industry as almost 90 % of the chemical industry is based on catalytic reactions. The development of new, more efficient catalysts will allow significant advances in chemical production, but still remains a rather challenging problem for materials scientists. Here, we have proposed and investigated a new composite photocatalyst based on WB5-x-WB2 and TiO2 for H2 evolution from aqueous ethanol solution and CO2 reduction under visible light irradiation (410 nm). New composite photocatalysts WB5-x-WB2/TiO2 were synthesized and characterized by X-ray diffraction, UV–vis spectroscopy, X-ray photoelectron spectroscopy, and high-resolution transmission electron microscopy. Photocatalytic measurements show that the maximum activity of the composite photocatalyst 4 and 23 times higher than the activity of unmodified TiO2 in CO2 reduction and H2 evolution, respectively. Density functional calculations of the proposed reaction are in agreement with the provided experiments confirming the higher efficiency of the modified catalyst compared to TiO2.
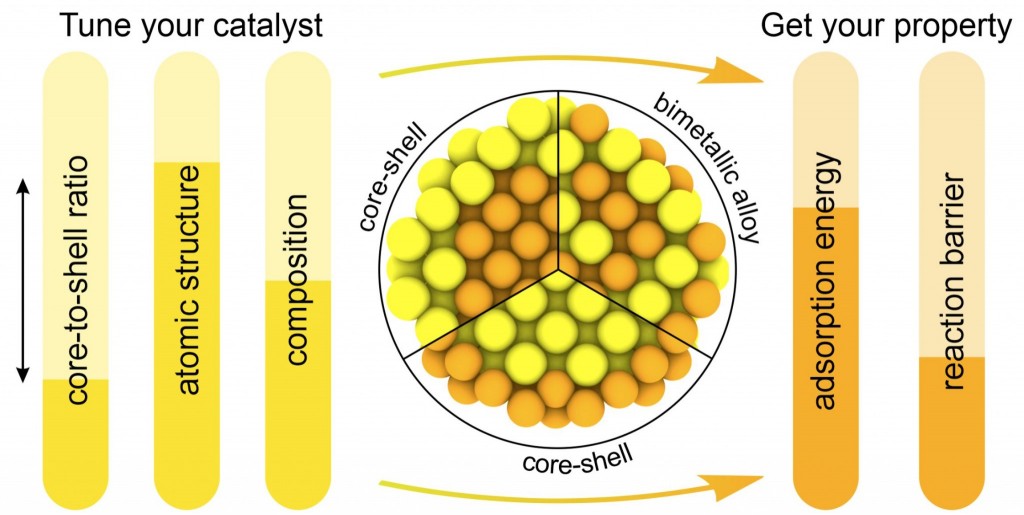
REVIEW. Structure-driven tuning of catalytic properties of core–shell nanostructures (Nanoscale, 2024, 16, 5870 – 5892)
The annual increase in demand for renewable energy is driving the development of catalysis-based technologies that generate, store and convert clean energy by splitting and forming chemical bonds. Thanks to efforts over the last two decades, great progress has been made in the use of core–shell nanostructures to improve the performance of metallic catalysts. The successful preparation and application of a large number of bimetallic core-shell nanocrystals demonstrates the wide range of possibilities they offer and suggests further advances in this field. Here, we have reviewed recent advances in the synthesis and study of core-shell nanostructures that are promising for catalysis. Particular attention has been paid to the structural tuning of the catalytic properties of core-shell nanostructures and to theoretical methods capable of describing their catalytic properties in order to efficiently search for new catalysts with desired properties. We have also identified the most promising areas of research in this field, in terms of experimental and theoretical studies, and in terms of promising materials to be studied.
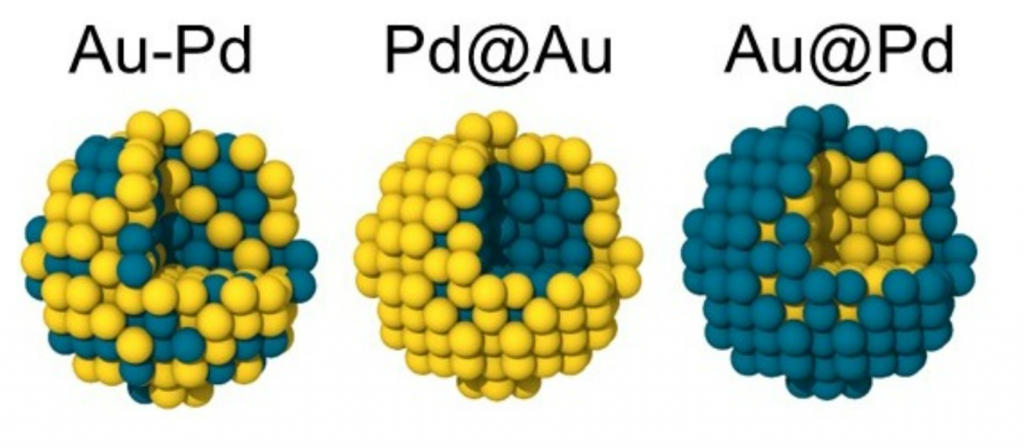
Tuning the surface properties of AuPd nanoparticles for adsorption of O and CO (Phys. Chem. Chem. Phys. 2023,25, 33031-33037)
Bimetallic nanoparticles are attracting increasing attention as effective catalysts because they can exhibit higher efficiencies than their monometallic counterparts. Recent studies show that PdAu nanoparticles can exhibit truly impressive catalytic activity, due to the synergistic effect of their properties. However, fine-tuning the catalytic activity requires an understanding of the full picture of the processes taking place in bimetallic particles of different compositions and structures. Here we study the influence of the structure and composition of PdAu nanoparticles on their electronic properties, charge distribution and adsorption properties (CO and O) using ab initio calculations. Two types of nanoparticles were considered: core–shell (Pd@Au and Au@Pd) and bimetallic alloy (Au–Pd) with an average diameter of 2 nm (321 atoms), having either fcc, icosahedral or amorphous structures. The results obtained on surface charges show the possibility of fine-tuning the surface properties of nanoparticles by modifying their atomic structure and composition. In addition, the adsorption of O and CO on the surface of PdAu nanoparticles with fcc structure has been studied. The obtained adsorption data correlate with the surface charge redistribution and the d-band center. The results of this study thus open up great prospects for tuning the catalytic properties of nanocatalysts by modifying their local atomic environment.
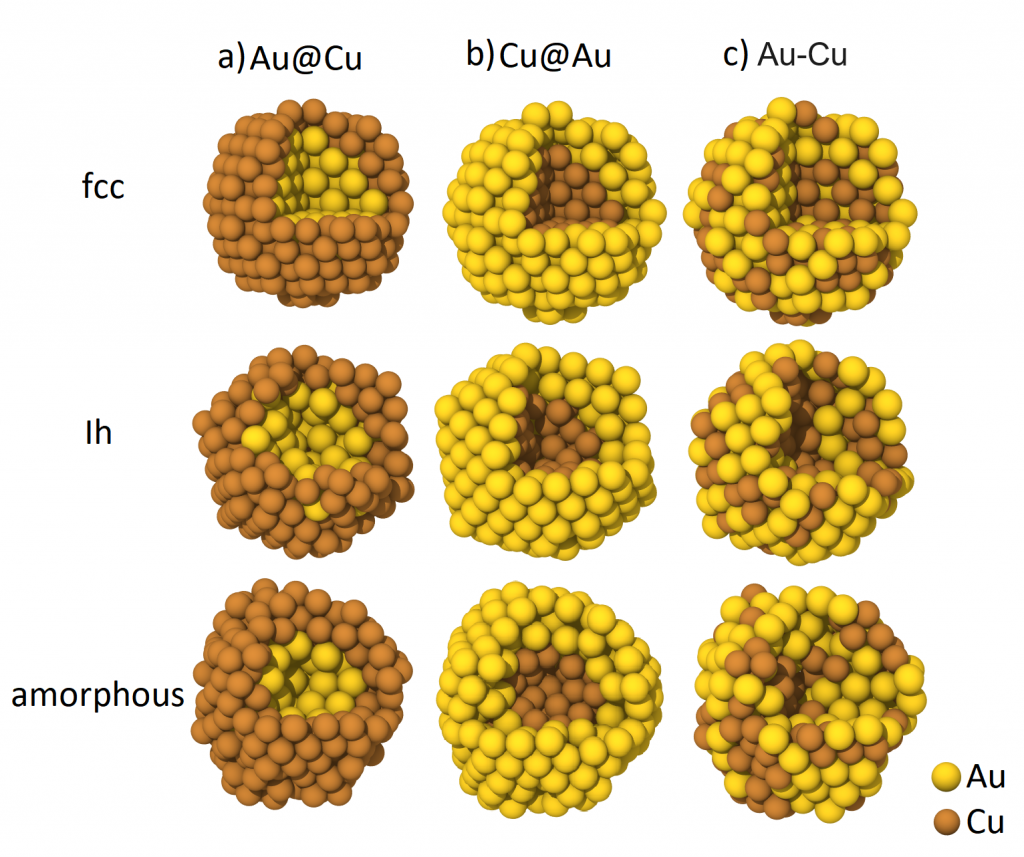
Structure-driven tuning of O and CO adsorption on AuCu nanoparticles: A density functional theory study (Phys. Rev. B, 2023, 108, 205414)
Nanoparticles are fascinating nanoobjects that are actively studied and widely employed in various fields, such as heterogeneous catalysis and advanced electrode materials. Their local atomic structure and composition can significantly impact their properties. We employed the state-of-the-art first-principles calculations to investigate the effects of AuCu nanoparticle structure and composition on the electronic properties, charge distribution, and CO and O adsorption energies. Two types of nanoparticles were examined in this study; specifically core-shell nanoparticles (Cu@Au, Au@Cu) and bimetallic alloy particles (Au-Cu), all with an average diameter of 2 nm (321 atoms) and exhibiting fcc, icosahedral, and amorphous structures. The research comprehensively investigated the electronic and adsorption properties of the proposed nanoparticles with regard to their ability to adsorb CO and O, revealing the potential for finely tuning of their properties by modifying their atomic structure and composition. Adjusting the core-to-shell ratio allows one to precisely tune the O and CO adsorption energies on the nanoparticle surface, particularly in fcc nanoparticles. This results in a narrower range of adsorption energies, specifically for CO adsorption, which cannot be achieved in bimetallic alloys. Our study shows the significance of this approach for fine-tuning adsorption energies on a nanoparticle surface.
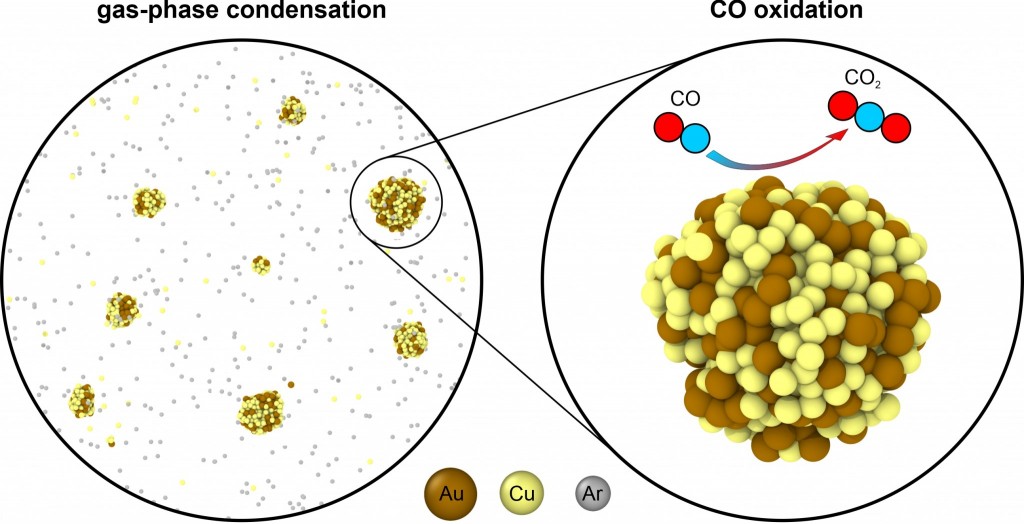
Cu–Au nanoparticles produced by the aggregation of gas-phase metal atoms for CO oxidation (Aggregate, 2022, e273)
Alloy nanoparticles (nanoalloys) are widely applied in heterogeneous catalysts, advanced electrodes, biomaterials, and other areas. Often such synthesis occurs in non-equilibrium conditions and yields nanoalloys with structures and properties that are different from those obtained in thermodynamic equilibrium. In this work, we characterize how the non-equilibrium conditions during the synthesis of Cu–Au alloys via physical vapor deposition (PVD) affect their morphology, composition, electronic structure, and reactivity in CO oxidation. We used molecular dynamics to simulate the PVD synthesis of Cu–Au nanoalloys through the non-isothermal aggregation of Cu and Au atoms at a 3:1 ratio in the Ar atmosphere to obtain realistic structures of Cu–Au nanoparticles. Due to the different aggregation kinetics of Au and Cu atoms, the average Au concentration in the obtained Cu–Au particles varied between 14% and 50% depending on the nanoparticle size and the aggregation time. Density functional simulations revealed that the reactivity of the obtained Cu–Au clusters toward CO and oxygen as well as Brønsted–Evans–Polanyi relations for CO oxidation significantly depend on whether the clusters had fcc, icosahedral, or amorphous structures and do not strongly correlate with the d-band centers of the adsorption sites. Our study highlights the importance of the non-equilibrium character of nanoalloy structure and composition for their electronic structure and catalytic properties. The performed analysis of the reactivity of Cu–Au clusters with realistic structures in CO oxidation will help the optimization of Cu–Au catalysts for this societally important reaction.